A unique Micro-Invasive Ultrasound guided BPH Solution*
BPH SOLUTION

EchoLaser® TPLA™
Transperineal Laser Ablation
APPLICATION
EchoLaser is used in case of patients suffering from Benign Prostatic Hyperplasia (BPH), with lower urinary tract symptoms.
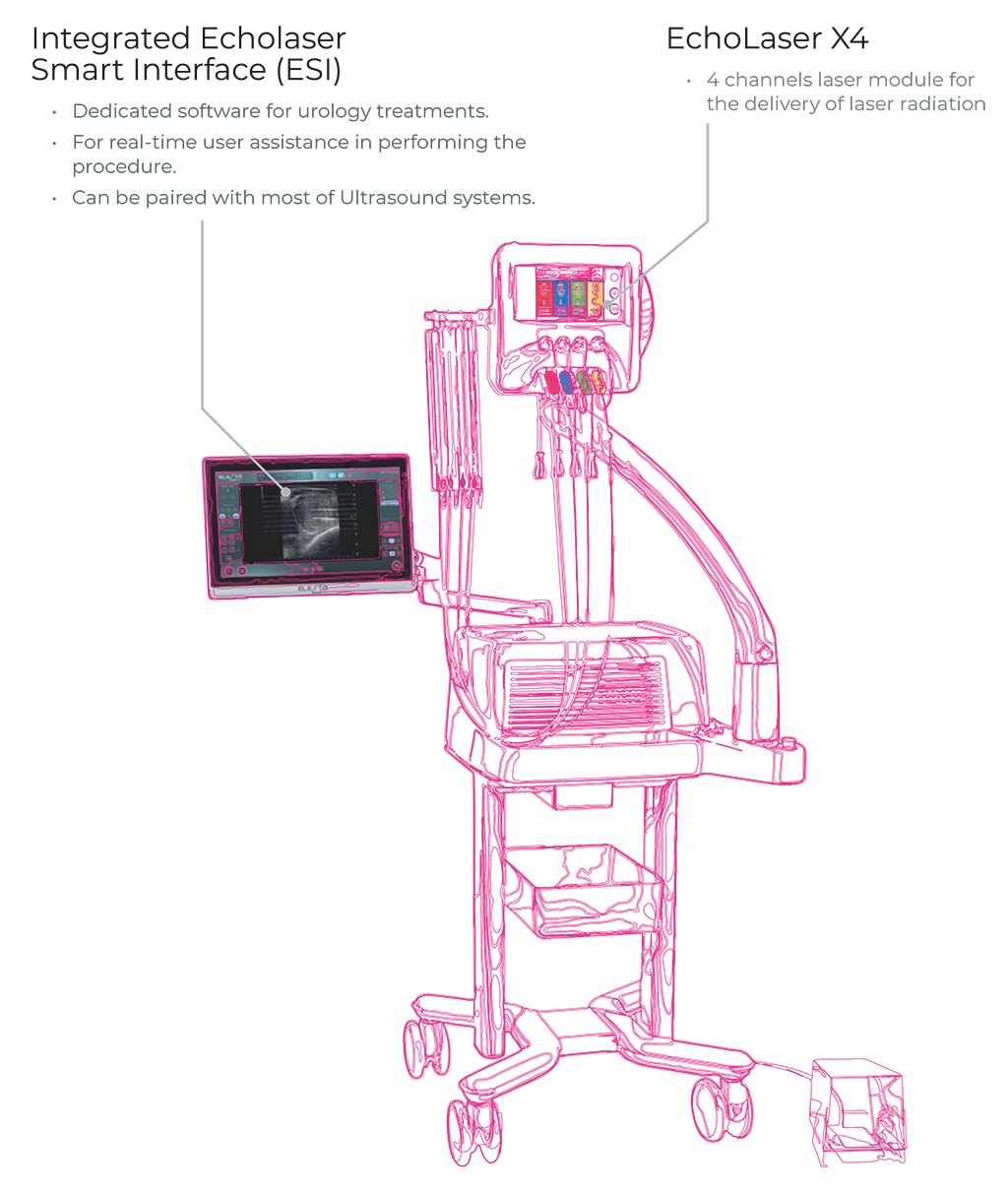
WHAT IS ECHOLASER?
Micro- Invasive technique with fine needles:
EchoLaser TPLA™ uses the laser light to heat the tissue to be treated until irreversible in-situ damage occurs.
Very fine needles (21G) are inserted via transperineal access without touching the urethra, for a treatment a-traumatic and very well tolerated by the patient.
Real-time monitoring of the treatment It can be performed under ultrasound
guidance for real-time monitoring of the correct positioning of the laser light applicators.
BENEFITS

• Ejaculation preservation (96%)
• Erectile function preservation
• Continence preservation
• Micro-invasive approach (thanks to the extremely fine needles)
• No need for a general anaesthesia
• Also suitable for high-risk patients
• Short duration of treatment (few minutes)
• No risk of urethral strictures/stenosis
• No or reduced post-op pain
• No or reduced hospitalisation
• Fast recovery times
MAIN BIBLIOGRAPHY
• Transperineal Laser Ablation of the Prostate for Symptomatic Benign Prostatic Hyperplasia: Long-Term Follow-Up in 40 Patients.
Patelli G, Altieri VM, Ierardi AM, Carnevale A, Chizzoli F, Baronchelli G, Trimarchi R, Carrafiello G.
Journal of Vascular and Interventional Radiology, 2024,ISSN 1051-0443, https://doi.org/10.1016/j.jvir.2024.04.023
• Transperineal laser ablation of the prostate as a treatment for benign prostatic hyperplasia and prostate cancer: The results of a Delphi consensus project.
Cocci A et al.
Asian Journal of Urology, 2023, ISSN 2214-3882, https://doi. org/10.1016/j.ajur.2023.07.001.
• Three years outcomes of transperineal laser ablation of the prostate.
Minafra P, DE Rienzo G, Gerbasi S, Cindolo L, Battaglia M, Ditonno P.
Minerva Urol Nephrol. 2023 Jun 14. doi: 10.23736/S2724- 6051.23.05270-9. Epub ahead of print. PMID: 37314812.
• Transperineal Laser Ablation for Benign Prostatic Enlargement: A Systematic Review and Pooled Analysis of Pilot Studies.
Tafuri A, Panunzio A, De Carlo F, Luperto E, Di Cosmo F, Cavaliere A, Rizzo M, Tian Z, Shakir A, De Mitri R, Porcaro AB, Cerruto MA, Antonelli A, Cormio L, Carrieri G, Karakiewicz PI, Abreu AL, Pagliarulo V.
J Clin Med. 2023 Feb 26;12(5):1860. doi: 10.3390/jcm12051860. PMID: 36902647; PMCID: PMC10003190.
• Ejaculatory Function following Transperineal Laser Ablation versus TURP for Benign Prostatic Obstruction: A Randomized Trial.
Bertolo R, Iacovelli V, Cipriani C, Carilli M, Vittori M, Antonucci M, Maiorino F, Signoretti M, Petta F, Travaglia S, Panei M, Bove P.
BJU Int. 2023 Mar 14. doi: 10.1111/bju.16008. PMID: 36917033.
• Trans-Perineal laser ablation of the prostate in high surgical risk patients affected by severe lower urinary tract symptoms related to benign prostatic obstruction.
Destefanis P, Sibona M, Vitiello F, Vercelli E, Micai L, Montefusco G, Mangione C, Bracco F, Colucci F, De Nunzio C, Gontero P.
Prostate Cancer Prostatic Dis (2023). https://doi.org/10.1038/s41391-023-00736-5
• Office-Based Transperineal Laser Ablation for Benign Prostatic Hyperplasia Under Local Anesthesia: 2-Year Results from a Dose Range Confirmatory Trial.
Bianco FJ, Luna E, Lopez-Prieto A, González P, Gheiler EL, Kaufman AM, Avila L, Maiolino G.
JU Open Plus 2(2):e00007, February 2024. | DOI: 10.1097/JU9.0000000000000105.
ECHOLASER® TPLA™ PROCEDURE
Before the procedure
• Position the patient in gynecological position
• Insert a bladder catheter to visualize the urethra
• Measurement of the prostate volume
• Transperineal local anesthesia (skin + periprostatic block)
Needle insertion with transperineal approach
• Definition of the positioning strategy for needles and fibers with ESI (EchoLaser Smart Interface) based on the morphology of the prostate
• Insertion of the needles: one or two 21G introducer needles for each lobe, according to the prostate dimensions and shape
• Removal of the inner part of the needle to allow the fiber insertion
Fiber Insertion
• Insert the 300-μm optical laser fiber into each needle
• Check safety distance of the fibers from the surrounding anatomical structures
and verify it with ESI
• Energy supply (5-10 min)
• Pull-back : if necessary based on the volume of the adenoma (5-10 min)
• Removal the needles + fibres
Patient discharged
Usually the same day of the procedure.
ECHOLASER® VIDÉO
SYSTEM COMPONENTS

Multi-needle guiding systems

Disposable fiber optics kit
- Fiber Optic
- Chiba Introduce
ECHOLASER® REFERENCES
EchoLaser®
| Reference | EchoLaser® system | Quantity / box |
| MEL01 | EchoLaser® | 1 |
| Reference | EchoLaser® Fiber Kits | Quantity / box |
| ROEL_KITX20 | Laser Thermal Therapy Kit LE | 20 |
EchoLaser® and TPLA™ are trademarks owned by Elesta
Products Specifications are subject to change without notice.
Please consult product label and insert for any indications, contraindications, hazards, warnings, cautions and directions for use.
For the latest information, always check the “Instructions for Use” that comes packaged with the product.
Bi-flex Evo
Ureteral Access Sheath


Flexible Atraumatic Tip
- The new Flexible Tip – Access to the meatus providing security and ensures an atraumatic path in all tortuous urinary tracts.
- Kink Resistant Coil – Steel spires within the inner coil provide strength and stability while allowing easy entry and withdrawal of the endoscope.

Graduated inner part
- Graduated Inner Part – Understand its position, in order to measure the dilation of the ureter with precision and safety.
- Distal Platinum Ring – Allows for visualization under fluoroscopy in order to confirm the location of the distal tip.
- Tapered introducer/Dilator – Tapers for an atraumatic introduction and dilation.

Bigger Inner Diameter
- « (…) The inner diameter of the 10/12 Bi-Flex is actually 11 F, so intrarenal pressures were lower and irrigation flow was higher (…) The irrigation flow measurements were in accordance with pressure measurements »*
*T.Sener, J.Cloutier, L.Villa, F.Marson, Buttice, S.Doizi and O. Traxer France Journal of Endourology, September 18, 2015
Bi-Flex Access Sheath
| References | Description | Ø Inner / Outer (Ch/Fr) | Length (cm) |
|---|---|---|---|
| ROUS1028ST | Bi-Flex Evo: Ureteral Access Sheath | 10/12 | 28 |
| ROUS1228ST | Bi-Flex Evo: Ureteral Access Sheath | 12/14 | |
| ROUS1035ST | Bi-Flex Evo: Ureteral Access Sheath | 10/12 | 35 |
| ROUS1235ST | Bi-Flex Evo: Ureteral Access Sheath | 12/14 | |
| ROUS1045ST | Bi-Flex Evo: Ureteral Access Sheath | 10/12 | 45 |
| ROUS1245ST | Bi-Flex Evo: Ureteral Access Sheath | 12/14 |
Individually Packaged / Sterile / Single Use Device / Box of 1.
